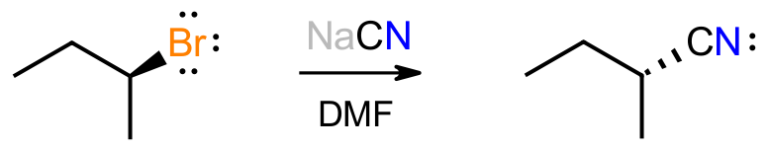key mechanisms - some general ideas
For “neutral” read inert or unreactive first in that the substrate itself is unlikely to be attacked as there are no strong acids or bases present. The substrate will have time to “do its own thing” for example by breaking apart in carbocation-based reactions. Consider the SN1 reaction between a tertiary alkyl halide in an alcohol solvent. The pH of the mixture will be neutral when the chemicals are first introduced so there is no strong acid to be attacked or strong base to do the attacking. The alkyl halide is able to collapse to a carbocation, which then attracts the electron-rich O of the alcohol and an ether is formed. In the example below, the stable carboxylic acid does not react with water (or alcohols) at neutral pH as there is nothing that reactive present.
If we can identify the conditions in a reaction as being acidic (H2SO4, HBr, generic H3O+, etc.) we already know what is going to happen first; the organic substrate is going to attack the acid and the substrate will become activated. This is of major importance in acid-catalyzed reactions where neutral conditions are not sufficient for reactivity but adding an acid gets things going by activating the organic substrate. This shows up in dozens of mechanisms in Organic 1 and 2 and gives an element of predictability to how pathways unfold. Since we are dealing with a “positive” environment, any intermediates formed during these pathways will be positively charged (oxonium ions, carbocations, etc.).
See the reaction bottom left for an example where the substrate attacks the acid to yield a cationic oxonium ion intermediate, which may react further depending on what else is present. The environment in the bottom right example has changed to strongly basic so the “attacker” and “attackee” change. Now the negative (electron-rich) base can attack the (electron-poor) carboxylic acid proton as a way to become stable through deprotonation. Notice how the mechanism arrows have changed direction in going from acidic to basic conditions. In general, organic substrates attack acids while bases attack organic substrates. This is a reliable indicator of what will happen in other mechanisms in the different pH environments.
While blanket generalities can often be dangerous, there are some ideas that can be used systematically to help us become comfortable with mechanism, especially in the second semester where the pathways become more detailed and more complicated. Knowing the main concepts behind Substitution, Elimination, and Addition from Organic 1 in detail will make Organic 2 that much more manageable.
Mechanisms will either be concerted or stepwise, they will involve reversible and/or irreversible processes, and they will be governed by the ideas of steric environment and/or electronic stability. There aren’t many options here so it is important to understand that the paths open to molecules in chemical reactions are fairly limited.
Substitution
Consider the reaction between the secondary alkyl bromide shown and sodium cyanide in DMF as a polar, aprotic solvent. We know that this gives the inverted product of substitution in which the cyanide anion has replaced the bromide. We also expect the new C-C bond to be stronger than the C-Br bond that is lost. So, what clues lead to the 2 arrow mechanism shown below?
Firstly, with HCN having a pKa of about 9.2, the reaction is moderately basic because of the cyanide salt, which suggests that the basic cyanide will attack the organic substrate. Since there are no obviously acidic protons on the substrate, there will be no acid-base reaction, and the negative cyanide will be attracted to the electron-poor alpha carbon next to Br.
We only have two choices for how this mechanism plays out; either everything happens at once in a concerted pathway, or the Br breaks off first in a stepwise pathway to give a carbocation, which then reacts with cyanide to give product. The second pathway would give enantiomeric products, i.e. a racemic mixture, because a flat carbocation would be formed. This does not happen so we focus on the concerted path.
The mechanism shown matches the paradigm described earlier. The basic reagent attacks the substrate, from the back where the antibond is, with the Br being displaced to maintain the octet at C. The negative charge has now been transferred from C to Br, which handles it better. This mechanism matches the experimental evidence, in which the stereochemistry is inverted, and the kinetic data that shows the reaction to be bimolecular.