Pushing Mechanism Arrows
-
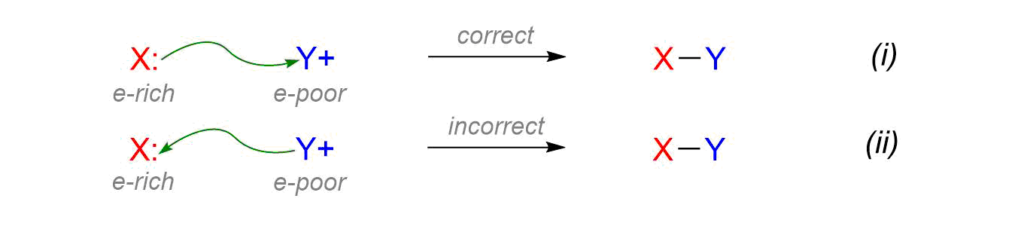
Organic mechanisms are simply a description of the bond-making and bond-breaking events that occur when starting materials are converted to products. The “curved arrows” used to describe these events were pioneered by Sir Robert Robinson in the UK and are now the convention used by chemists throughout the world. While undergraduate students often struggle with this idea it is as simple as driving on the correct side of the road; curved arrows in polar mechanisms always start at electron-rich areas and are always pushed towards electron-poor areas.
If we think of electron density as a currency then the idea of “electron-rich” donating to “electron-poor” makes sense as long as we can identify sites of electron excess and electron deficiency. In Equation (i) the green curved arrow is used correctly to describe the lone pair from X (electron-rich) being used to form a covalent bond to Y (electron-poor). The green arrow in Equation (ii) is incorrect because Y does not have any extra electron density to share with X. In most of the reactions we study a curved arrow will start at a lone pair or pi bond and be pushed towards an electron-poor atom.
-
Beginning with an acid-base reaction (Equation iii) we assess what bonds have been formed and broken and focus on the atoms involved. In this equation a new bond is formed between N and H and a bond is broken between H and O. The sodium ion does not change and is simply a spectator ion. The placement of the Na next to N on the left-hand side does tell us which atom will be the base.
-
N is negatively charged and therefore reactive (Equation iv). While the O in water also has two lone pairs, the O is neutral and much less reactive than a negative N. The N atom will try to become neutral by attacking water, in particular at one of the H atoms since they are electron-poor due to the electronegativity difference between O and H. This results in N becoming neutral and the negative charge being transferred to the more electronegative O.
-
To complete the mechanism for this process we must add curved arrows to describe all bonds being formed and broken during the reaction. The arrows must start at an electron-rich area and go towards an electron-poor area. Here the N is the aggressive electron-rich species so an arrow starts there and goes to H to form the N-H bond. To avoid breaking the octet rule, an arrow goes from the O-H bond to the O atom. Two events, two arrows.
-
If we assess what needed to be done in the acid-base example we can extrapolate that to organic mechanisms since the number of curved arrows will (typically) match the number of bonds that need to be formed and broken. The first carbon-based mechanism usually studied in the Organic sequence is the bimolecular substitution of a nucleophile on an electrophile with loss of leaving group. This actually boils down to one bond being formed and one being broken. Considering Equation (vi) it appears that the C-CN bond must form and the C-Br bond must break.
-
As with the acid-base reaction we identify the electron-rich species (cyanide anion) and the electron-poor species (C in the methyl group). One bond needs to form and one needs to break so, knowing that this reaction is bimolecular and concerted, the nucleophile is going to kick out the leaving group on carbon as described in Equation (vii). This simple preliminary analysis of what needs to happen in each reaction will take a lot of the mystery out of mechanism.